■ BCG ワクチン接種による新型コロナウイルス感染症(COVID-19)発症抑制効果の可能性と自然免疫トレーニング(自然免疫記憶)についての考察
自然免疫制御技術研究組合
研究本部長・稲川裕之
新型コロナウイルス感染症(COVID-19)の発症抑制とBCG ワクチン接種との因果関係に関する議論がインターネットを中心として活発に行われている。この関係は興味深いが、根拠が明確でなかった。この関係について査読前の論文が公開され、根拠の一端が示された。さらに、別の論文においてBCG ワクチン接種が持つウイルスや細菌などの感染予防効果は“自然免疫のトレーニング”とか“自然免疫記憶”といわれる機構で説明できる可能性が報告されている。そこで、自然免疫を活性化するLPS(lipopolysaccharide)にも自然免疫のトレーニング効果がある可能性について考察する。
『BCG ワクチン接種とCOVID-19 発症との疫学研究』
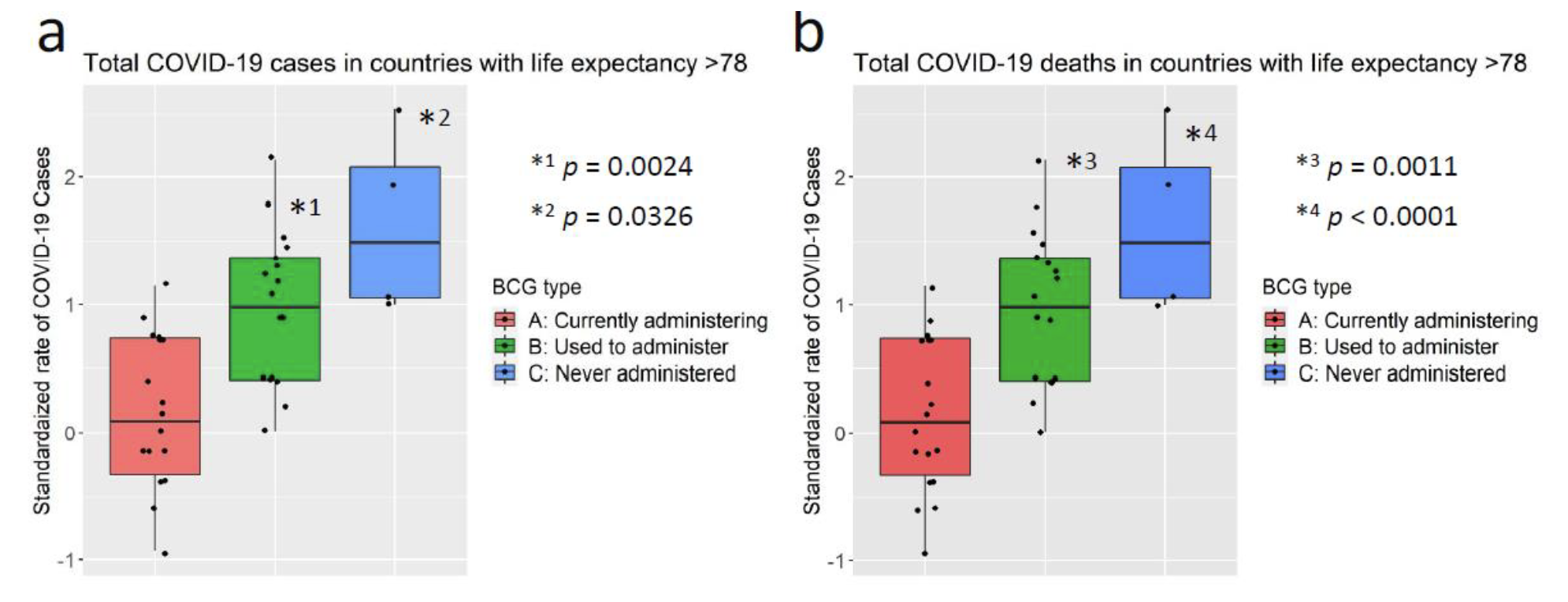
「BCG ワクチン接種がCOVID-19 発症抑制に関連する」根拠の一端を示す論文が『medRiv』(査読前の論文を掲示するサイト)『Association of BCG vaccination policy with prevalence and mortality of COVID-19』https://doi.org/10.1101/2020.03.30.20048165 に公開された。本論文は2020 年3 月20 日時点のデータに基づいてCOVID の発症抑制とBCG 接種との関連性を解析している。本論文の図を下に引用する。図は世界各国を一つの点として表し、ここ2 ヶ月の平均気温、平均寿命とCovid-19 の感染者数、死亡者数との関連性を解析している。この図からCOVID-19 は気温とはほとんど関係がない。一方、平均寿命が長い国ほど感染率も死亡率も高くなることが強く示されている。
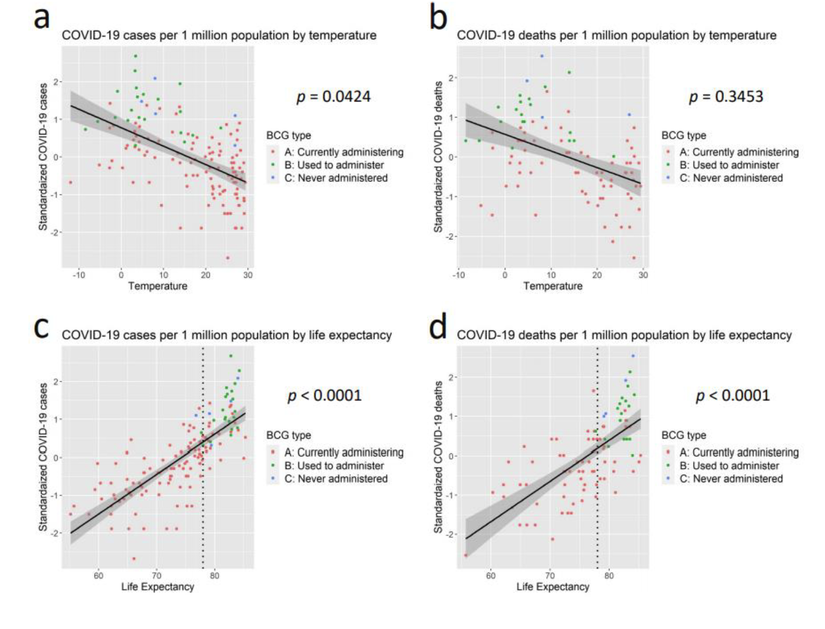
(a): 100 万人あたりのCOVID-19 症例数と2-3 月の平均気温との相関(弱い逆相関)
(b): 100 万人あたりのCOVID-19 死亡者数と2-3 月の平均気温との相関(相関なし)
(c): 100 万人あたりのCOVID-19 症例数と平均余命(強い相関有り)
(d): 100 万人あたりのCOVID-19 死亡者数と平均余命(強い相関有り)
さらに、筆者らはBCG ワクチン接種がCOVID-19 発症抑制に及ぼす影響を、平均寿命が78 歳を超える国に限定して解析している。BCG ワクチン接種について、各国をA: 通常BCG ワクチンを接種している国、B:BCG ワクチンを接種していた経験のある国、C: BCGワクチン未接種国のグループに郡分けすると、100 万人あたりの症例数と死亡数が共にA:BCG ワクチンを接種している国<B:BCG ワクチンを接種していた経験のある国<C:BCGワクチン未接種国、ときれいに分けられる。つまり、高齢化が進んでいる国においてBCG ワクチン接種国では非接種国に比べて、感染者数も死亡数も明らかに少ない。確かに他の関連因子との関わりが明確になっていない現状では、BCG ワクチン接種がCOVID-19 発症抑制に効果があるとの結論を出すのは時期尚早であろう(https://doi.org/10.1101/2020.04.08.20056051.t)。しかしながら、BCG ワクチン接種とCOVID-19 発症抑制との関係がデータで明確に示されたことは、BCG のCOVID-19 発症抑制を含む感染症予防効果メカニズムを研究する上で、重要な基盤となりうると思われる。
『BCG ワクチンや自然免疫活性化物質の自然免疫トレーニング効果』
BCG ワクチン接種が結核菌以外の感染症を予防する効果は自然免疫の活性化に基づくことが示唆されており、このことについて、既に複数の研究報告がある。BCG によるCOVID19発症抑制という現象は、その根本に“自然免疫のトレーニング”とか“自然免疫記憶”というユニークな機構が関係すると言われているが、関連してこれらの研究を取りまとめた最近の総説であるNature Reviews Immunology (2020 年3 月)『Defining trained immunity and its role in health and disease』https://doi.org/10.1038/s41577-020-0285-6 を紹介したい。
この論文では、一度自然免疫の活性化が起こると、二度目の後のウイルスや細菌などの感染に対して、一度目の感染より高い活性化が起こること、すなわちBCG ワクチン、βグルカン、LPS などの自然免疫の活性化物質は“trained immunity”を誘導できる可能性があること、それがエピジェネティックな遺伝子制御に基づくこと、が示されている。
下図はそのイメージ図であり、縦軸は自然免疫の活性、横軸はPrimary stimulation、Secondary stimulation を含む時間軸、上半分がTrained immunity、下半分はtolerance(自然免疫の活性化低下)が示されている。今回はTrained immunity のみ説明するが、Primary stimulation がBCG ワクチン接種、Secondary stimulation は新型コロナウイルス感染として考えてもらうとわかりやすい。BCG ワクチン接種で最初に自然免疫が一旦活性化される時(一回目の山)に、免疫細胞の中でエピジェネティクな遺伝子変化が起こり、その結果として自然免疫記憶が残る。次の刺激であるコロナウイルスが感染した時に、自然免疫の活性化が高くなり(二回目の山)、自然免疫がトレーニングされたとされている。なお、BCG の生ワクチンでは5 年(少なくとも3 ヶ月から1 年)はトレーニングされた自然免疫の潜在能力(つまり二回目の刺激があると自然免疫の活性が高くなること)が持続するとのことである。(なお、BCG を接種したのは殆どの人は5 年以上前であるが、微量の結核菌刺激を受け続けている場合はそのメモリー効果が持続する可能性がある。)
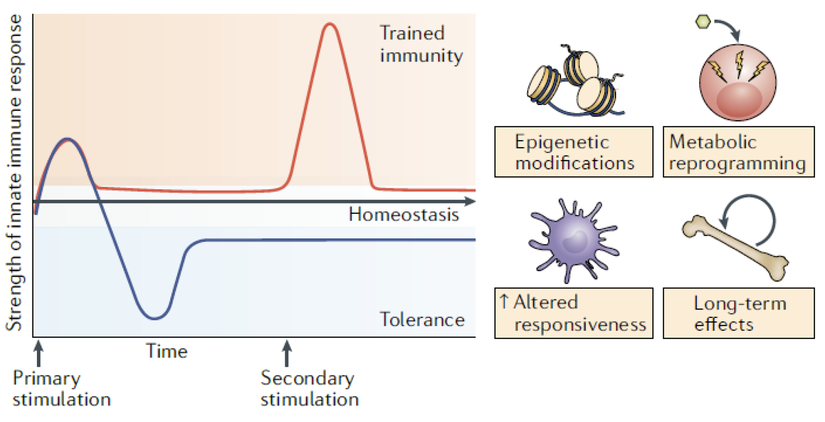
このメカニズムは部分的にしか理解されていないが、いくつかのレベル(遺伝子変化、longnon-coding RNA(lncRNAs)、DNA メチル化、細胞代謝の再プログラミング)の関与が示されているとのことである。詳細は原著を参照頂きたい。
『LPS の自然免疫トレーニング能力』
さて、この論文では、LPS にはこの自然免疫をトレーニングする機能があることが示唆されているが、細菌やウイルスなどの感染症予防効果についての記載がない。そこで、LPS が自然免疫をトレーニングし、感染症を予防する機能を持つことに関する論文を調べたところ、理化学研究所のグルーブが2015 年8 月にNature Immunology に『The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory』doi:10.1038/ni.3257 という論文を発表していた。
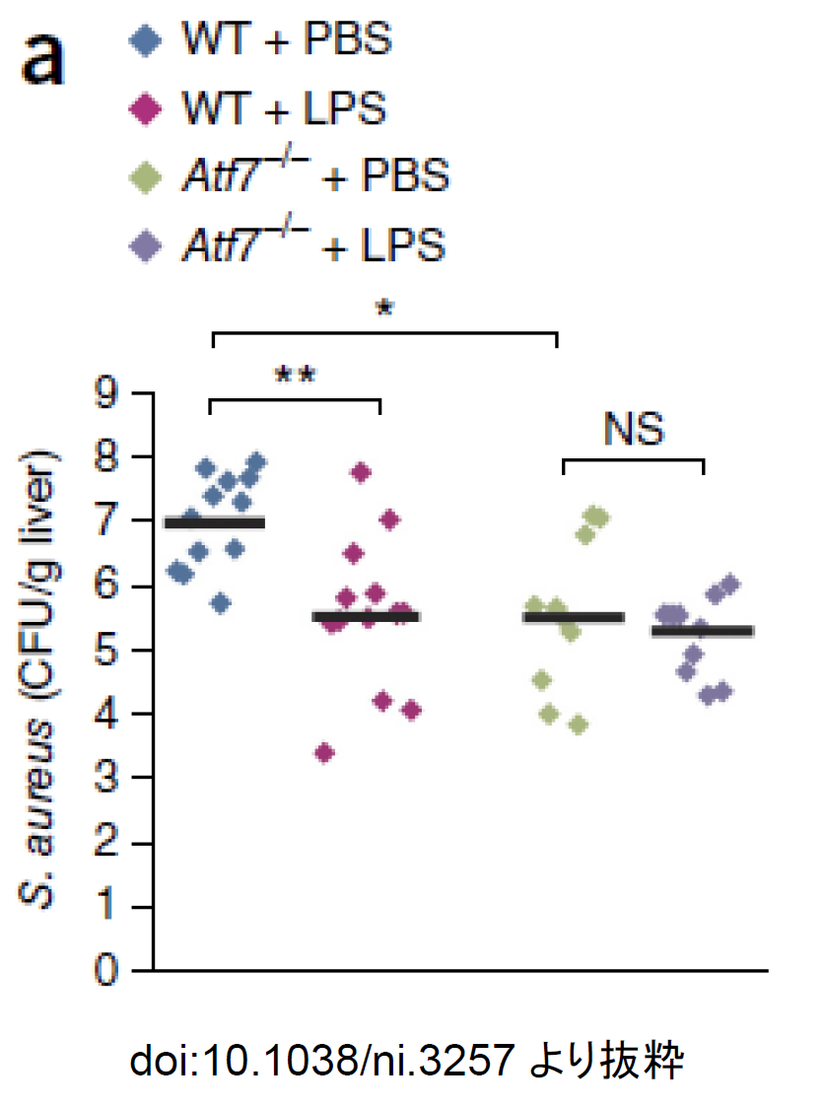
本論文では、LPS でマクロファージを活性化すると、ATF7 という転写因子を介して免疫系遺伝子のエピジェネティックな変化誘導され、それが自然免疫記憶として3 週間持続することが示されている。また、この機構が衛生仮説やワクチンのアジュバント作用にも関わると筆者らは述べている。
本論文で、LPS が自然免疫をトレーニングする機能があることを示す具体的なデータを引用する。下の図は、マウスに細菌を感染(黄色ブドウ球菌注射)させて、肝臓中の細菌数を調べたもので、LPS を注射(腹腔内で3 週間前)すると、LPS 注射していないマウスの10 分の1 に細菌数が低下している。ATF7 ノックアウトマウスではその効果が見られていないことから、LPS の3 週間前の投与による感染予防効果はATF7 の関与が示されている。
本論文ではLPS は腹腔内に注射する実験系を用いているが、通常ではLPS が腹腔内に投与されるなどということはなく、ヒトは普段、経口、経皮でLPS を摂取している。しかも、これまでの研究から経口投与したLPS は、抗アレルギー、がん治療の補助的効果、異物排除(貪食)能の向上、抗体産生のアジュバント作用増強など多くの有用な効果を発現することが示されている。(Ann Agric Environ Med.2016; 23(2): 206–222. doi: 10.5604/12321966.1203879)。しかし、経口投与したLPS が自然免疫をトレーニングする機能についての報告はまだ無いようである。けれども、LPS をマウスの鼻腔内に7 日前に投与しておけばインフルエンザウイルスに対する予防効果が得られる報告(J Virology, 2011, http://dx.doi.org/10.1128/JVI.06168-11)があることを見れば、粘膜投与したLPS は自然免疫をトレーニングする機能を持ち、自然免疫記憶を誘導する機能を持つことが示唆される。今後、経口投与したLPS は自然免疫をトレーニングする機能を持つことについて現象面は言うまでもなく、分子レベルでそのメカニズムを明らかにしていきたいと考えている。

Pantoea agglomerans: a mysterious bacterium of evil and good. Part IV. Beneficial effects
Annals of Agricultural and Environmental Medicine 2016, Vol 23, No 2,206-222
Jacek Dutkiewicz(1), Barbara Mackiewicz(2), Mart a Kinga Lemieszek(3), Marcin Golec(1), Janusz Milanowski(2)
(1) Department of Biological Health Hazards and Parasitology, Institute of Rural Health, Lublin, Poland
(2) Department of Pneumonology, Oncology and Allergology, Medical University, Lublin, Poland
(3) Department of Medical Biology, Institute of Rural Health, Lublin, Poland
Abstract
Pantoea agglomerans, a gammaproteobacterium of plant origin, possesses many beneficial traits that could be used for the prevention and/or treatment of human and animal diseases, combating plant pathogens, promotion of plant growth and bioremediation of the environment. It produces a number of antibiotics (herbicolin, pantocins, microcin,agglomerins, andrimid, phenazine, among others) which could be used for combating plant, animal and human pathogens or for food preservation. Japanese researchers have demonstrated that the low-molecular-mass lipopolysaccharide of P. agglomerans isolated by them and described as 'Immunopotentiator from Pantoea agglomerans 1 (IP-PA1)' reveals the extremely wide spectrum of healing properties, mainly due to its ability for the maintenance of homeostasis by macrophage activation. IP-PA1 was proved to be effective in the prevention and treatment of a broad range of human and animal disorders, such as tumours, hyperlipidaemia, diabetes, ulcer, various infectious diseases, atopic allergy and stress-induced immunosuppression; it also showed a strong analgesic effect. It is important that most of these effects could be achieved by the safe oral administration of IP-PA1. Taking into account that P. agglomerans occurs commonly as a symbiont of many species of insects, including mosquitoes transmitting the Plasmodium parasites causing malaria, successful attempts were made to apply the strategy of paratransgenesis, in which bacterial symbionts are genetically engineered to express and secrete anti-Plasmodium effector proteins. This strategy shows prospects for a successful eradication of malaria, a deadly disease killing annually over one million people, as well as of other vector-borne diseases of humans, animals and plants. Pantoea agglomerans has been identified as an antagonist of many plant pathogens belonging to bacteria and fungi, as a result of antibiotic production, competition mechanisms or induction of plant resistance. Its use as a biocontrol agent permits the decrease of pesticide doses, being a healthy and environmental-friendly procedure. The application of the preparations of this bacterium efficiently protects the stored pome, stone and citrus fruits against invasion of moulds. P. agglomerans strains associated with both rhizosphere and plant tissues (as endophytes) efficiently promote the growth of many plants, including rice and wheat, which are the staple food for the majority of mankind. The promotion mechanisms are diverse and include fixation of atmospheric nitrogen, production of phytohormones, as well as degradation of phytate and phosphate solubilizing which makes the soil phosphorus available for plants. Accordingly, P. agglomerans is regarded as an ideal candidate for an environmental-friendly bioinoculant replacing chemical fertilizers. It has been documented that the Pantoea strains show biodegradation activity on various chemical pollutants of soil and water, including petroleum hydrocarbons and toxic metals. P. agglomerans prevents the penetration of harmful industrial contaminants into deeper parts of soil by biofilm formation, and has an ability to produce hydrogen from waste. Thus, this bacterium appears as a valuable bioremediator which, in some cases, may be acquired as a cheap form of energy. In conclusion, in spite of the proven pathologic role of P. agglomerans in causing occupational diseases of allergic and/or immunotoxic background and accidental infections, the beneficial traits of this species, and of related species of Pantoea genus, are of great value for potential use in many areas of biotechnology. Hence, any restrictions on the use of these organisms and their products should be declined, providing safety precautions at work with the Pantoea biopreparations are maintained.
Key words
Pantoea agglomerans, beneficial effects, LPS, immunostimulation, treatment of tumors, healing properties, vector-borne diseases, paratransgenesis, biocontrol of plant pathogens, antibiotics, plant growth promotion, bioremediation
Clinical Effects of Orally Administered Lipopolysaccharide Derived from Pantoea agglomerans on Malignant Tumors
Anticancer Research 36:3747-3752 (2016)
ATSUTOMO MORISHIMA(1) and HIROYUKIINAGAWA(2)(3)
(1)Lavita Medical Clinic, Hiradai-cho, Moriguchi-shi, Osaka, Japan;
(2)Faculty of Medicine, Kagawa University, Miki-cho, Kita-gun, Kagawa, Japan;
(3)Research Institute for Healthy Living, Niigata University of Pharmacy and Applied Life Sciences, Niitsu-shi, Niigata, Japan
Abstract
Background/Aim: It has been reported that oral administration of lipopolysaccharide (LPS) recovers an individual's immune condition and induces the exclusion of foreign matter, inflammation and tissue repair. We orally administered LPS from the wheat symbiotic bacteria Pantoea agglomerans, which has been ingested and proven to be safe, to cancer patients. Our observation of clinical improvements resulting from this treatment are reported. Patients and Methods: Sixteen cancer patients who exhibited declined small intestinal immune competence were treated between June and September, 2015. Diagnosis was based on our evaluation on small intestinal immune competence and macrophage activity.
Results: The state of malignant tumors at 3 months after starting this treatment was complete recovery for 3 cases, remission for 7 cases, maintenance for 4 cases, exacerbation for 1 case and death for 1 case (total response rate=62.5%). Small intestinal immune competence and macrophage activity recovered in all cases, suggesting that oral administration of LPS contributes to disease improvement. No clear side-effects that appeared to be related to LPS intake were noted.
Conclusion: Intake of an appropriate level of Pantoea agglomerans LPS recovers small intestinal immune competence and macrophage activity, contributing to improvement of malignant tumors' therapy.
In recent years, it has been reported that oral administration of lipopolysaccharide (LPS) activates immune cells through toll-like receptor (TLR) 4, inducing foreign body exclusion, inflammatory action and tissue repair action (1). We reported that a hot water extract of wheat flour (oral administration) contains macrophage-activating substances derived from concomitant Gram-negative plant-associated bacteria, such as Pantoea agglomerans. LPS of this bacterium is a major macrophage-activating substance (2, 3). It has been reported that Pantoea agglomerans were found to be symbiotic in many plants, such as wheat and brown rice (4, 5). Oral administration of LPS from Pantoea aggomerans amplified phagocytic activity of peritoneal macrophages through TLR 4 (6). It has been reported in animal models and clinical studies of humans that oral intake of LPS from Pantoea agglomerans can prevent the onset of type I diabetes, control blood glucose levels in type II diabetes and decrease low-density lipoprotein (LDL) cholesterol (7, 8). Furthermore, when treating a mouse melanoma transplantation model with an anti-carcinogenic agent (doxorubicin), oral administration of Pantoea agglomerans LPS improves the survival of the B16 melanoma-inoculated murine model (9). Therefore, oral administration of LPS is thought to lead to superior improvement in treatment outcomes (9).
At our hospital, we have implemented immunity-improving therapies in the form of intestinal flora using kampo medicine or lactic acid bacteria supplements. However, as these mainly recover functioning of the large intestine and do not recover small intestinal competence, they do not completely increase immunity. To sufficiently increase immunity, small intestinal immune competence also needs to be recovered. Therefore, we focused on the substance called LPS, which recovers small intestinal immune competence.
Here, we used LPS from wheat symbiotic bacteria, Pantoea agglomerans, which has been ingested and confirmed as safe.
In the present article, we report on the effects observed in our evaluation of the oral administration of LPS to 16 patients with malignant tumors who requested alternative therapy.
Intradermal administration of lipopolysaccharide in treatment of human cancer
Cancer Immunol Immunother (1996) 42: 255 –261
Abstract
Lipopolysaccharide (LPS) has been recognized as a potent antitumor agent in animal tumor models; however, its use in human cancer therapy has been limited to only one trial, in which LPS from Salmonella was given intravenously. It was not very successful because of poor tumor response and was also toxic. We originally developed LPS prepared from Pantoea agglomerans (LPSp), and this was a well-purified, small-molecular-mass (5 kDa) agent.
We chose intradermal rather than intravenous administration in the hope that the former would release LPS slowlyinto the bloodstream, and thus be less toxic while preserving antitumor activity. In our animal tumor models, intradermal administration was indeed less toxic and more beneficial for tumor regression than intravenous administration.
We made a pilot study with intradermal administration of LPSp on the treatment of ten advanced cancer patients. Five of them had evaluable tumor, which had failed earlier to respond to conventional chemotherapy.
Cyclophosphamide was also administered in this trial, in anticipation of its synergistic effect with LPSp. In this study LPSp was injected intradermally into each patient twice a week, starting with an initial dose of 0.4 ng/kg, and raising it to 600 or 1800 ng/kg. A 400-mg/m2 dose of cyclophosphamide was given intravenously every 2 weeks. After completion of the dose escalation, the treatment was continued for at least 4 months, and it was found that
1800 ng/kg LPSp was well tolerated. A significant level of cytokines was observed in the sera for at least 8 h. These results indicate higher tolerable doses and remarkably more continuous induction of the cytokines than were reported in a previous study by others using intravenous administration.
Three of the five evaluable tumors showed a significant response to our combined therapy. Intradermally administered, LPS was less toxic and elicited a tumor response in combination with cyclophosphamide; it can thus can be applied to cancer treatment even in humans.
Antitumor Mechanism of Intradermal Administration of
Lipopolysaccharide
ANTICANCER RESEARCH 17: 1961-1964 (1997)
Abstract
We earlier demonstrated that 50 % of the lethal dose of lipopolysaccharide (LPS) from Pantoea agglomerans given by the intrademal (i.d.) route is about 300 times greater than that given by the intravenous (i. v.) route, and that 400 μg/kg of LPS administered i.d. significantly suppressed metastasis whereas administered iv. it did not. To learn the specific mechanism involved in this i.d. administration, the fate of LPS at the skin following administration and the concurrent production of endogenous tumor necrosis factor (TNF) in serum was examined. Histological observation following the i.d. administration of LPS (40 μg/kg) revealed neutrophiles in the skin 6 hours later. After 24 or 48 hours inflammatory cells were assembled at the site of injection. Endogenous TNF activity was found in the skin 24 hours after the injection and was significantly detectable even after 48 hours. Endogenous TNF was induced around tumor lesions of Meth A fibrosarcoma, MH134 hepatoma and Lewis lung carcinoma by treatment of LPS administered i.d. Taken together, these findings suggest that the antitumor activity of i.d. administered LPS results from the continuous supply of a small amount of this substance producing free TNF and activating inflammatory cells such as macrophages having membrane bound proTNF on their surface from the injected site to the tumor lesion for more than 48 hours.
We reported that lipopolysaccharide (LPS) an endotoxin of Gram negative bacteria, when administered intradermally (i.d.) had an antitumor effect against an allogenic murine tumor model (1) and syngeneic murine tumors, Meth A fibrosarcoma, MH 134 hepatoma and Lewis lung (LL) carcinoma model (2,3), and cancer patients (4) without severe side effects. Intradermal (i.d.) or oral administration of LPS decreased ils toxicity without lessening the curative effect on various intractable diseases (5,6). ln a comparison of the inhibitory effect of lung metastasis of Meth A to the mouse by LPS administered i.d. or intravenously (i.v.), a significant inhibition was observed in i.d. treated mice. whereas this was not true in mice treated i.v. (2). Although an effective dose of LPS by the i.d. route against the syngeneic tumor model was comparable to that by the i.v. route (3,7), the LD50 of i.d. LPS administration is about 300 times higher than that of i.v. administration (2).
These results suggested that a different mode of action was involved in the antitumor effect by the two routes.
Three observations were noted in an attempt to understand the characteristics of the antitumor effect following i.d. administration of LPS. The first is that carbon clearance in blood was enhanced to the same level as by the i.v. route, and accumulation of inflammatory cells was noted in the healing lesions (1). The second is that the antitumor effect of the i.d. administration was neutralized by anti-TNF serum (2). The third is that we proved that i.d. administration of LPS efficiently induced endogenous TNF in vivo (8). These findings suggested that this administration route induced the activation of the inflammatory cells (macrophages, neutrophiles and others) that produce a moderate amount of free tumor necrosis factor (TNF) or the expression of membrane-bound TNF (precursor TNF) on activated macrophages (9,10), which are known to have a cytocidal effect (11,12) in the host body.
We then hypothesized that i.d. adminjstered LPS was localized in the skin lesion and gradually released into the bloodstream from the skin, and exerting its antitumor effect by activating inflammatory cells.
We discuss here the putative mechanism of action of i.d. administration of Pantoea agglomerans LPS (LPSp).
学会発表データ(詳細)
- 1990 Intratumoral induction of tumour necrosis factor by systemic administration of Bordetella pertussis vaccine.pdf
- 1992 Homeostasis as regulated by activated macrophage. I. Lipopolysaccharide (LPS) from wheat flour isolation, purification and some biological activities.pdf
- 1992 Homeostasis as Regulated by Activated Macrophage. II. LPS of Plant Origin Other than Wheat Flour and Their Concomitant Bacteria.pdf
- 1992 Homeostasis as Regulated by Activated Macrophage. III. Protective Effect of LPSw (Lipopolysaccharide (LPS) of Wheat Flour) on Gastric Ulcer in Mice as Compared with Those of Other LPS from Various Sourc.pdf
- 1992 Homeostasis as regulated by activated macrophage. IV. Analgesic effect of LPSw, a lipopolysaccharide of wheat flour.pdf
- 1992 Homeostasis as regulated by activated macrophage. IX. Enhancement effect of LPSw (a lipopolysaccharide from wheat flour) on hen egg-laying and breaking strength of eggshell.pdf
- 1992 Homeostasis as Regulated by Activated Macrophage. V. Suppression of Diabetes Mellitus in Non-obese Diabetic Mice by LPSw (a Lipopolysaccharide from Wheat Flour).pdf
- 1992 Homeostasis as Regulated by Activated Macrophage. VI. Protective Effect of LPSw (a Lipopolysaccharide from Wheat Fluor) against Acute Infection by Toxoplasma gondii in Mice.pdf
- 1992 Homeostasis as Regulated by Activated Macrophage. VII. Suppression of Serum Cholesterol Level by LPSw (a Lipopolysaccharide from Wheat Flour) in WHHL (Watanabe Heritable Hyperlipidemic) Rabbit.pdf
- 1992 Homeotasis as regulated by activated macrophage. VIII. LPSw (a lipopolysaccharide from wheat flour) can regulate bone resorption of chick embryo.pdf
- 1992 Oral or percutaneous administration of lipopolysaccharide of small molecular size may cure various intractable diseases a new version of Coley's toxin.pdf
- 1993 further developments of the therapy with lipopolysaccharides of a small molecular size on various intractable diseases.pdf
- 1994 Enhanced production of tumour necrosis factor alpha (TNF-alpha) by its precursor on the cell surface of primed THP-1 cells.pdf
- 1995 Immunomodulator as medicine for morphine and cocaine dependence. Especially effect of LPS.pdf
- 1996 Antitumor mechanism of intradermal administration of lipopolysaccharide.pdf
- 1996 Antitumor mechanism of intradermal administration of lipopolysaccharide_20190720044954318.pdf
- 1996 Intradermal administration of lipopolysaccharide in treatment of human cancer.pdf
- 1997 Antitumor mechanism of intradermal administration of lipopolysaccharide.pdf
- 1997 Structural characterization of lipid A obtained from Pantoea agglomerans lipopolysaccharide.pdf
- 1998 INVOLVEMENT OF 26-kDa MEMBRANE-BOUND TUMOUR NECROSIS FACTOR PRECURSOR IN BIDIRECTIONAL FEEDBACK REGULATION ON 17-kDa TUMOUR NECROSIS FACTOR PRODUCTION AFTER STIMULATION BY LIPOPOLYSACCHARIDE.pdf
- 1998 Mechanisms by which Chemotherapeutic Agents Augment the Antitumor Erects ofTumor Necrosis FactorInvolvement ofthe Pattern Shift of Cytolttnes from Th2 to Th1 in Tumor Lesions.pdf
- 2005 Innate-immune Therapy for Lung Carcinoma Based on Tissue-macrophage Activation with Lipopolysaccharide.pdf
- 2006 Applications of Lipopolysaccharide Derived from Pantoea agglomerans (IP-PA1) for Health Care Based on Macrophage Network Theory.pdf
- 2006 Inherent potential for production of tumor necrosis factor-α by human intestinal macrophages.pdf
- 2006 Specific messenger RNA expression for signal transduction molecules by lipopolysaccharide in intestinal macrophages.pdf
- 2006 Unique Molecular Characteristics of the Environmental Responses of Mucosal Macrophages.pdf
- 2007 Development and potential use of a monoclonal antibody to the lipopolysaccharide of Pantoea agglomerans (IP-PA1).pdf
- 2008 Preventative and Therapeutic Potential of Lipopolysaccharide Derived from Edible Gram-Negative Bacteria to Various Diseases.pdf
- 2008 Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits.pdf
- 2009 Improvement of Allergic Dermatitis via Regulation of the Th1Th2 Immune System Balance by Macrophages Activated with Lipopolysaccharide Derived from Pantoea agglomerans (IP-PA1).pdf
- 2009 Intestinal Macrophages Involved in the Homeostasis of the Intestine Have the Potential for Responding to LPS.pdf
- 2009 Mechanism for Maintaining Homeostasis in the Immune System of the Intestine .pdf
- 2009 ROS and Innate Immunity.pdf
- 2009 Utility and Safety of LPS-based Fermented Flour Extract as a Macrophage Activator.pdf
- 2010 Antitumor Effect of Inhalatory Lipopolysaccharide and Synergetic Effect in Combination with Cyclophosphamide .pdf
- 2010 Cytotoxic Effects of Activated Alveolar Macrophages on Lung Carcinoma Cells viaCell-to-Cell Contact and Nitric Oxide .pdf
- 2010 Preparation of Lipopolysaccharide Derived from Pantoea agglomeransLabeled with Fluorescence as a Tracer for Kinetics Analysis .pdf
- 2010 Protective Effects of the Immunopotentiator from Pantoea agglomerans 1 on Chemotherapeutic Agent-induced Macrophage Growth Inhibition.pdf
- 2010 Recovery from Immunosuppression-related Disorders in Humans and Animals by IP-PA1, An Edible Lipopolysaccharide.pdf
- 2011 A mixture of Salacia oblonga extract and IP-PA1 reduces fasting plasma glucose (FPG) and low-density lipoprotein (LDL) cholesterol levels.pdf
- 2011 Evaluation of the Acute Toxicity, Irritation, Hypersensitivity and Genetic Toxicity for Lipopolysaccharides from Pantoea agglomerans (LPSp) .pdf
- 2011 Functional Characterization of Lipopolysaccharide derived from Symbiotic Bacteria in Rice as a Macrophage-activating Substance.pdf
- 2011 Oral administration of immunopotentiator from Pantoea agglomerans 1 (IP-PA1) improves the survival of B16 melanoma-inoculated model mice.pdf
- 2011 Oral administration of lipopolysaccharides for the prevention of various diseases benefit and usefulness.pdf
- 2012 Lipopolysaccharide-Induced Microglial Activation and Neuroprotection against Experimental Brain Injury Is Independent of Hematogenous TLR4..pdf
- 2012 The Involvement of O-Antigen Polysaccharide in Lipopolysaccharide in Macrophage Activation.pdf
- 2014 Pantoea agglomerans lipopolysaccharide maintains bone density in premenopausal women a randomized, double-blind, placebo-controlled trial.pdf
- 2014 Uncovering potential “herbal probiotics” in Juzen-taiho-to through the study of associated bacterial populations.pdf
- 2015 A Lipopolysaccharide from Pantoea Agglomerans Is a Promising Adjuvant for Sublingual Vaccines to Induce Systemic and Mucosal Immune Responses in Mice via TLR4 Pathway.pdf
- 2016 Clinical Effects of Orally Administered Lipopolysaccharide Derived from Pantoea agglomeranson Malignant Tumors .pdf
- 2016 Effects of 3 months continuous intake of supplement containing Pantoea agglomerans LPS to maintain normal bloodstream in adults Parallel double- blind randomized controlled study.pdf
- 2016 Pantoea agglomerans Part IV. Beneficial effects.pdf